Investigation Into Millet for Use as an Eco-Friendly Intumescent Coating for Passive Fire Protection
By: Zara Stefanie Vermeulen, MEng graduate (supervisors: N Flores Quiroz, R Walls, University of Stellenbosch). This project was funded by an SFPE student award.
View full PDF here
Introduction
Intumescent paints or coatings are a popular form of passive fire protection in the fire industry. Intumescent paint development has been at the forefront of the passive fire protection industry for several years, with these paints and coatings now considered a reliable form of passive fire protection due to extensive research in predicting their thermal behaviour. These coatings typically form a carbonaceous layer when exposed to heat to provide a thermal barrier to structural elements. Thoubehaviorgh they are used on a global scale, studies have shown that intumescent paints release considerable toxic products during combustion, which can lead to health and environmental hazards [1]. As such, the impact of intumescent paint’s toxicity is an important consideration for sustainability, and whether this can be reduced with the inclusion of greener materials should be studied.
Past testing in literature [2 – 4] and scoping work has identified that certain biomass materials exhibit intumescent properties. This finding will be used as a foundation to study the intumescent properties of these biomass materials and the development of a biomass intumescent paint. This work focused on the intumescent performance of the agricultural crop known as millet, with specific emphasis on the physical characteristics and thermal resistance development of the grain. The material underwent three testing methods to determine its applicability as the main component in a sustainable intumescent paint, namely, thermogravimetric analysis (TGA), hot stage analysis, (HS) and cone calorimetry (CC).
Materials and methods
The primary biomass material analysed in this investigation was the Zimbabwean finger millet variety. Millet is a small-seeded agricultural food grain known for its advantageous properties. Zimbabwean finger millet is described as having a brick red colour, with a grain diameter of approximately 1.6 mm. Zimbabwean finger millet is presented in Fig. 1.

Fig. 1. Zimbabwean finger millet.
Thermogravimetric analysis (TGA) provided in-depth insight into the thermal degradation of the agricultural grain when exposed to elevated temperatures, with regards to the degradation stages and material components. One sample of the whole finger millet grain and one sample of the ground finger millet grain was tested in an air environment. Hot stage analysis (HS) provided visual snapshots of the material degradation and associated release of compounds. This allowed for comparison between TGA and HS for prediction of the grain behaviour in small-scale.
One sample of the singular whole finger millet was tested under various conditions: (1) sample in chamber with lid open (due to viewing window becoming misty when closed), and (2) sample immersion in silicone oil (for observation of gases released from the material). Cone calorimetry (CC) was conducted to provide insight into the thermal resistance development and performance of the grain, as well as visual and physical characteristics observed, on a larger scale. This is important for translation of the thermal behaviour from small-scale to larger scale experiments, for a singular finger millet grain compared to millet as one entity. CC tests were performed on whole finger millet in a standard cone calorimeter.
Results and discussion
Through comparison of the three experimental methods, the thermal resistance development of finger millet over time was found to occur in three respective phases, namely, inert phase, transient phase, and steady state and charred phase. The inert phase takes place from ambient conditions to the activation point, otherwise known as, the minimum temperature for the grain to intumesce. The transient phase occurs from the activation point to end of reaction, at which maximum expansion occurs, and the intumescent process comes to an end. The last phase, steady state and charred phase, considers further thermal degradation of the grain during which maximum heat transfer through the sample occurs.
The overall thermal behaviour of finger millet is summarised in Fig. 2. Further descriptions for each phase are presented below Fig. 2. In this work, it was assumed that negligible differences occur between critical points in the experimental methods, so that the overall behaviour of millet between the three methods can be assumed. In reality, some variation will most probably occur between the temperature readings of HS and CC.
Fig. 2: Schematic diagram presenting the overall thermal behaviour of whole finger millet as an intumescent material.
The inert phase takes place from 25 °C to 243 °C. The inert phase considers the agricultural grain prior to the intumescent process, during which the grain must first heat up to reach the minimum expansion temperature to initiate the intumescent chemical reactions. The outer layer of the grain must first breakdown before moisture can be released, after which, water vapour is released from the sample. During this phase, initial breakage of bonds also occurs so that thermal degradation can occur in the transient phase. At the end of this phase, there has been sufficient build-up of water vapour (inherent moisture) in the grain pericarp, resulting in failure of the pericarp and sudden expansion of the endosperm.
The transient phase occurs over the intumescent process of the agricultural grain, from 243 °C to 370 °C. The further degradation observed during the analysis in this work as compared to the popping process [5,6] can most likely be attributed to the grain being exposed to much higher temperatures, allowing for a larger pressure difference and breakdown of additional layers in the grain. Due to this pressure build up, volatiles are released which ultimately results in the breakdown of the germ layer beyond the stress limit of the grain, resulting in a rapid increase in volume. The produced volatiles and gases are composed of volatile emissions, ignition, burning into the flame, and flame extinction. Hence, a cellular matrix is produced presenting a black multi-cellular, honeycomb-like structure, with micro-pores. This process continues until all volatiles and gases are gone, and the final product is a carbon rich charred layer. The expansion of millet taking place can be associated with the degradation of cellulose, and due to the high cellulose content of millet, high expansion is observed.
The maximum expansion for HS and CC was 1.25 : 1 and 3 : 1, respectively. The higher expansion in the cone can be attributed to the formation of the lattice structure consisting of air bubbles or pockets between the grain particles. As millet undergoes further degradation, minimal volatiles are released, and the char structure reduces slightly in volume to ultimately result in a compact structure with limited air pockets. The expansion process as observed in the cone calorimetry tests can be seen in Table 1.
Table 1: Behaviour of whole finger millet over the cone calorimeter test duration with regards to visual and physical characteristics.
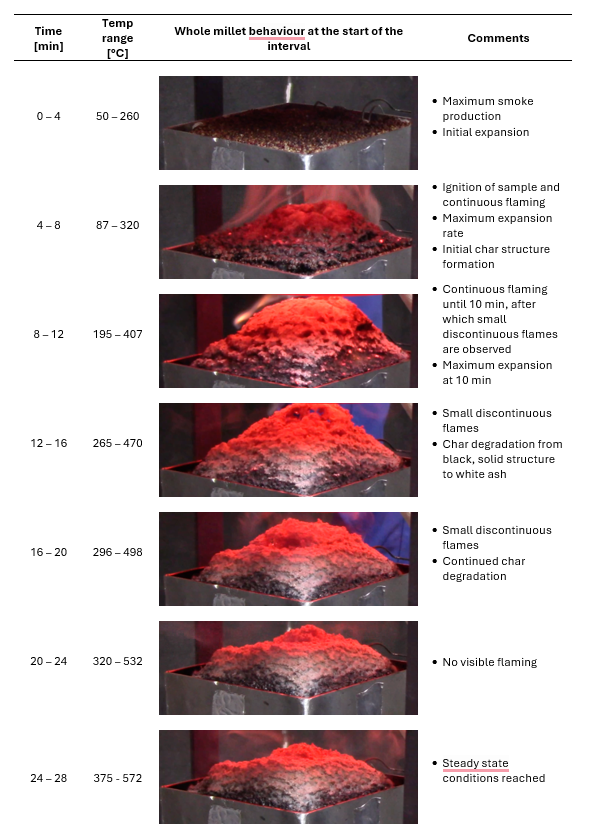
The steady state and charred phase considers the grain as having undergone full expansion and the entire coating as a charred surface. This phase takes place from 370 °C to the end temperature (600 °C in this work). During this phase, no additional reactions occur, and maximum heat transfer occurs through the sample due to no further expansion. The developed char structure continues to degrade further, changing from black/grey to white in colour, which can be attributed to the consumption of char. Fig. 3 visually describes the intumescent process of finger millet, ultimately resulting in an expanded charred layer.
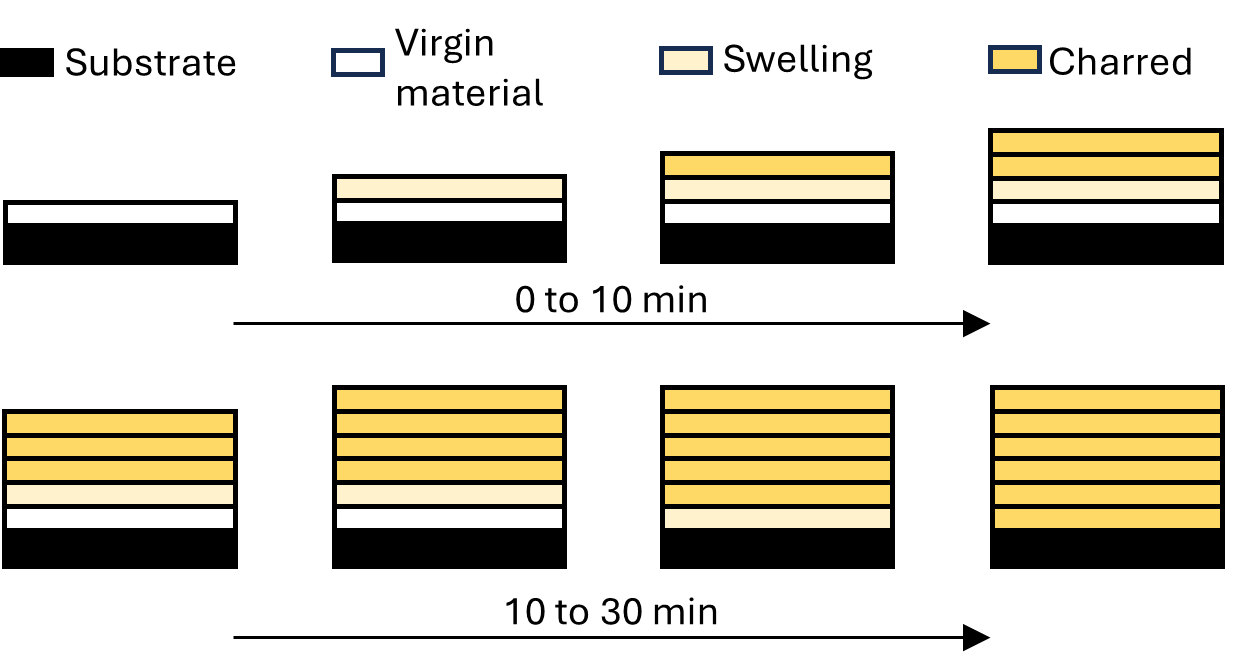
Fig. 3: Increase in discretized elements in millet over the intumescent process.
Conclusions
This work was able to predict the intumescent behaviour of millet, to provide a scientific basis to allow the promising behaviour to potentially be used in studying additional agricultural grains in the future. The grain presented good potential to be used as a form of passive protection, through the development of the lattice-like expanded structure and providing sufficient levels of insulation. This work provided the baseline for further studying of biomass materials for intumescent potential, with a wide array of topics of interest in future research.
References
[1] Sowriraajan, A.V. and Bhaskar Dixit, C.S. (2010) Development of environmentally benign fire retardant coatings. Bengaluru, India.
[2] Oguaka, A., Flores Quiroz, N. and Walls, R. (2023a) ‘Comparative pyrolysis characteristics and kinetics of agricultural food grains by thermogravimetric analysis’, Process Safety and Environmental Protection, 179, pp. 559–574. Available at: https://doi.org/10.1016/j.psep.2023.09.047.
[3] Oguaka, A., Flores Quiroz, N. and Walls, R. (2023b) ‘Fire parameters, behaviour, and comparative thermal hazard of food grains based on the cone calorimeter tests’, Process Safety and Environmental Protection, 179, pp. 928–940. Available at: https://doi.org/10.1016/j.psep.2023.03.078.
[4] Oguaka, A.B.C. (2023) Pyrolysis, ignition and fire behaviour of agricultural food grains. Stellenbosch University.
[5] Mishra, G., Joshi, D.C. and Kumar Panda, B. (2014) ‘Popping and puffing of cereal grains: A review’, Journal of Grain Processing and Storage, (2), pp. 34–46. Available at: www.jakraya.com/journal/jgps.
[6] Swarnakar, A.K., Mohapatra, M. and Das, S.K. (2022) ‘A review on processes, mechanisms, and quality influencing parameters for puffing and popping of grains’, Journal of Food Processing and Preservation, 46(10). Available at: https://doi.org/10.1111/jfpp.16891.